- 28/06/2024
- Posted by: Gaetan Dermien
- Category: Uncategorized

EU and GB approval changes (January-May 2024)
| EU and GB approval changes COLEAD’s Regulation Monitoring covers both European Union (EU)and Great Britain (GB) approval changes. Note that EU approvals still apply in Northern Ireland. Great Britain is the mainland comprising England, Scotland and Wales. The European Commission (EC) has recently published changes to 48 plant protection product (PPP) approvals within the EU, some of which are important for ACP horticulture. These include:
In addition, the EC has recently notified the WTO of its intention to approve the active substance metconazole and to withdraw the approval of acibenzolar-S-methyl. The Health and Safety Executive (HSE) has recently published changes to 44 PPP approvals within GB, of which 21 are of importance for ACP horticulture. These include:
How will ACP producers/exporters be affected? Non-approval, withdrawal of approval or expiration of approval mean that EU/GB maximum residue levels (MRLs) are likely to be maintained or reduced to the limit of determination (LoD) which, in most cases, will mean that they cannot be used on crops for export to the EU/GB. !! NEW!! COLEAD now compiles a dashboard of ACP countries impacted by the recent changes of approvals in the EU and GB. Check it out here.
What should ACP producers/exporters do now? If you currently use dodemorph or Trichoderma atroviride strain IMI 206040 on crops destined for the EU market, or one of the withdrawn substances from GB on crops for the GB market, you need to look for alternatives, or ensure that current uses allow you to comply with the new MRLs (most likely at LoD), as soon as the change is communicated. If this is likely to cause you significant problems, and you fear being left without effective and available alternatives, please contact COLEAD at: network@colead.link. We will keep you informed as more information becomes available. |
Our news is a quarterly update designed to inform you of any changes introduced during the preceding five months. If you require more frequent updates on EU MRL changes, we recommend that you visit our AGRINFO website and subscribe to the bi-monthly newsletter on EU regulations.
ACP countries potentially impacted by changes of EU and GB PPP approvals during the period January-May 2024
COLEAD has compiled a dashboard presenting registered products containing the active substances for which the approval is not renewed or withdrawn in the EU and in GB and their respective conditions of use in ACP countries. This aims to outline the ACP countries where these approval changes might have repercussions for export. It is based on information extracted from the latest national lists of registered plant protection products made available by 34 ACP countries (list available here).
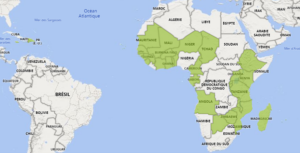 Figure 1: Map of the potentially impacted ACP countries following the changes of approvals in the EU and GB
Figure 1: Map of the potentially impacted ACP countries following the changes of approvals in the EU and GB
COLEAD is making all effort to provide you with relevant and updated information. If your country is not included in the 34 countries included in our review, we highly recommend reaching out to your national authorities to check which products might be affected by these changes.
Key changes of approval in the EU during the period January-May 2024
The EC has recently published changes to 48 plant protection product (PPP) approvals within the EU. In this News, ‘key active substances’ refers to those used/registered in one or more ACP countries on horticultural crops that are frequently exported regionally or internationally. While COLEAD makes every effort to provide comprehensive information about EU and GB PPP regulatory changes, it is possible that some PPPs or crops relevant to you are not included in our list of key substances/crops. We recommend that you review the following section, which gives details on all approval changes since the beginning of 2024, to check for any others that could affect you. If you see any PPP that you use on crops for export to the EU or GB in the lists below, we recommend that you check the regulation itself using the link provided.
Approval periods for the following key substances have been extended (new expiry dates):
- buprofezin (Expiry date: 15 December 2025)
- fluazinam (Expiry date: 15 April 2026)
- fluopyram (Expiry date: 30 June 2026)
- lambda-cyhalothrin (Expiry date: 31 August 2026)
- pyraclostrobin (Expiry date: 15 September 2025)
- azadirachtin (Expiry date: 31 January 2027)
- bupirimate (Expiry date: 31 January 2027)
- dithianon (Expiry date: 31 January 2027)
- dodine (Expiry date: 15 July 2026)
- hexythiazox (Expiry date: 31 January 2027)
- tebufenozide (Expiry date: 31 January 2027)
Non-renewal of the active substances dimethomorph
Dimethomorph is a systemic fungicide effective against various fungal pathogens, such as downy mildew and late blight, and is commonly used on various crops such as cucurbits, onions, potatoes, and small fruits.
The European Commission (EC) has published Commission Implementing Regulation (EU) 2024/1207 concerning the non-renewal of approval of dimethomorph for use in the EU. This is due to concerns about dimethomorph’s toxicity to reproduction and its endocrine disruption properties in humans and mammals. Any grace period granted for use in the EU will expire by 20 May 2025. The non-renewal is expected to lead to a reduction in the dimethomorph maximum residue levels (MRLs) to 0.01–0.05 mg/kg on all products in 2025.
Expiration of the approval of the active substance dodemorph and Trichoderma atroviride strain IMI 206040
The EC has published Commission Implementing Regulation (EU) 2024/1280 concerning the expiration of approval of various active substances for use in the EU, due to the absence of application for renewal and support from the manufacturer. Among these, two are key to ACP horticulture: dodemorph and Trichoderma atroviride strain IMI 206040, both expiring on 31 August 2024.
All PPP approval changes in the EU introduced in 2024
Table 1 shows all changes to PPP approvals introduced in the EU since the beginning of 2024.
If you require additional information or face particular problems as a result of these changes, please contact COLEAD at: network@colead.link.
Key changes of approval in GB during the period January-May 2024
Since the beginning of 2024, approval status have been updated for 44 plant protection product (PPP) approvals within GB, of which 21 are considered key for ACP horticulture.
Approval periods for the following 11 key substances have been extended (new expiry dates):
- Aluminium phosphide (Expiry date: 31 August 2026)
- Magnesium phosphide (Expiry date: 31 August 2026)
- Bixafen (Expiry date: 31 May 2026)
- Fluxapyroxad (Expiry date: 31 May 2026)
- Buprofezin (Expiry date: 31 January 2031)
- Cyflufenamid (Expiry date: 31 March 2031)
- Penthiopyrad (Expiry date: 31 May 2026)
- Pyrethrins (Expiry date: 31 August 2026)
- Sulfoxaflor (Expiry date: 18 August 2026)
- 1,4-Dimethylnaphthalene (Expiry date: 30 June 2026)
- Mancozeb, extended twice, in January and in April (Expiry date: 30 May 2024)
Approval of the active substance pydiflumetofen
The Health and Safety Executive (HSE) has published its decision to approve the active substance pydiflumetofen for use in GB. Pydiflumetofen is a broad-spectrum fungicide used on various field crops to treat a wide range of plant diseases such as powdery mildew, septoria, cercospora leaf spot, and grey mould.
Withdrawal of the approvals for the following 9 key active substances:
- Oxamyl (withdrawn on 31 January 2024)
- Triflumuron (withdrawn on 31 March 2024)
- Abamectin (withdrawn on 30 April 2024)
- Trichoderma atroviride (formerly T. harzianum) strains IMI 206040 and T11 (withdrawn on 30 April 2024)
- Carboxin (withdrawn on 31 May 2024)
- Cyproconazole (withdrawn on 31 May 2024)
- Diethofencarb (withdrawn on 31 May 2024)
- Mancozeb (withdrawn on 31 May 2024)
- Myclobutanil (withdrawn on 31 May 2024)
All PPP approval changes in GB since the beginning of 2024
Table 2 shows all changes to PPP approvals introduced in GB since the beginning of 2024.
Proposed future approval changes
All draft regulations must be notified either to the WTO Sanitary and Phytosanitary Committee (SPS) or Technical Barriers Committee (TBT) for a commenting period of 60 days before they are adopted. This provides an important opportunity for countries to comment on the proposed changes, and to seek an amendment if a change is likely to have a significant impact on exports. To provide feedback stakeholders should contact their national WTO contact point .
References
Lewis, K.A., Tzilivakis, J., Warner, D. and Green, A. (2016). An international database for pesticide risk assessments and management. Human and Ecological Risk Assessment: An International Journal, 22(4): 1050-1064. DOI: 10.1080/10807039.2015.1133242
This publication has been developped by the Fit For Market Plus programme, implemented by COLEAD within the framework of Development cooperation between the Organisation of African, Caribbean and Pacific States (OACPS), and the European Union (EU). This publication has been produced with the financial support of the EU and the OACPS. Its contents are the sole responsibility of COLEAD and can under no circumstances be regarded as reflecting the position of the EU or the OACPS.





![EU and GB approval changes (January-March 2025) 9-FFM+-[ENG]](https://news.colead.link/wp-content/uploads/2024/06/9-FFM-ENG-150x150.jpg)