- 06/05/2025
- Posted by: Gaetan Dermien
- Category: Uncategorized

| EU and GB approval changes COLEAD’s Regulation Monitoring covers both European Union (EU)and Great Britain (GB) approval changes. Note that EU approvals still apply in Northern Ireland. Great Britain is the mainland comprising England, Scotland and Wales. The European Commission (EC) has recently published changes to 31 plant protection product (PPP) approvals within the EU, 12 of which concern the extension of approval for PPPs important for ACP horticulture. In addition, the EC has recently notified the WTO of its intention not to renew the approval for the active substance flufenacet. The Health and Safety Executive (HSE) has recently published changes to 10 PPP approvals within GB, including the withdrawal or approval of the active substance metaflumizone, which is of importance for ACP horticulture.
How will ACP producers/exporters be affected? Non-approval, withdrawal of approval or expiration of approval mean that EU/GB maximum residue levels (MRLs) are likely to be maintained or reduced to the limit of determination (LoD) which, in most cases, will mean that they cannot be used on crops for export to the EU/GB.
What should ACP producers/exporters do now? If you currently use dodemorph or flufenacet on crops destined for the EU market, or metaflumizone on crops for the GB market, you need to look for alternatives or ensure that current uses allow you to comply with the new MRLs (most likely at LoD), as soon as the change is communicated. If this is likely to cause you significant problems, and you fear being left without effective and available alternatives, please contact COLEAD at: network@colead.link. We will keep you informed as more information becomes available. |
Our news is a quarterly update designed to inform you of any changes introduced during the preceding three months. If you require more frequent updates on EU approval changes changes, we recommend that you visit our AGRINFO website and subscribe to the bi-monthly newsletter on EU regulations.
ACP countries potentially impacted by changes of EU and GB PPP approvals during the period January-March 2025
COLEAD has compiled a dashboard presenting registered products containing the active substances for which the approval is not renewed or withdrawn in the EU and in GB and their respective conditions of use in ACP countries. This aims to outline the ACP countries where these approval changes might have repercussions for export. It is based on information extracted from the latest national lists of registered plant protection products made available by 34 ACP countries (list available here).
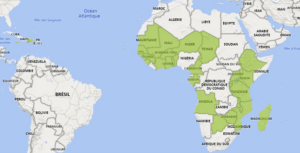 Figure 1: Map of the potentially impacted ACP countries following the changes of approvals in the EU and GB in 2025
Figure 1: Map of the potentially impacted ACP countries following the changes of approvals in the EU and GB in 2025
COLEAD is making all effort to provide you with relevant and updated information. If your country is not included in the 34 countries included in our review, we highly recommend reaching out to your national authorities to check which products might be affected by these changes.
Key changes of approval in the EU during the period January-March 2025
The EC has recently published changes to 31 plant protection product (PPP) approvals within the EU. In this News, ‘key active substances’ refers to those used/registered in one or more ACP countries on horticultural crops that are frequently exported regionally or internationally.
Approval periods for the following key substances have been extended (new expiry dates):
- Benalaxyl-M (Expiry date: 30 September 2027)
- Cyprodinil (Expiry date: 31 October 2026)
- Fosetyl (Expiry date: 31 October 2026)
- Halosulfuron-methyl (Expiry date: 15 November 2026)
- Milbemectin (Expiry date: 31 May 2026)
- Phenmedipham (Expiry date: 30 September 2026)
- Pirimicarb (Expiry date: 31 October 2026)
- Pyrimethanil (Expiry date: 30 June 2026)
- Pyriofenone (Expiry date: 30 June 2027)
- Spinosad (Expiry date: 31 October 2026)
- Sulphur (Expiry date: 31 July 2026)
- Triticonazole (Expiry date: 31 January 2027)
While COLEAD makes every effort to provide comprehensive information about EU and GB PPP regulatory changes, it is possible that some PPPs or crops relevant to you are not included in our list of key substances/crops. We recommend that you review the following section, which gives details on all approval changes since the beginning of 2025, to check for any others that could affect you. If you see any PPP that you use on crops for export to the EU or GB in the lists below, we recommend that you check the regulation itself using the link provided.
All PPP approval changes in the EU introduced in 2025
Table 1 shows all changes to PPP approvals introduced in the EU since the beginning of 2025.
If you require additional information or face particular problems as a result of these changes, please contact COLEAD at: network@colead.link
Key changes of approval in GB during the period August-November 2024
Since the beginning of 2025, approval status have been updated for 10 plant protection product (PPP) approvals within GB, of which 2 are considered key for ACP horticulture (metaflumizone and glyphosate).
Withdrawal of the active substance metaflumizone
The Health and Safety Executive (HSE) has decided to withdraw the approval the active substance metaflumizone for use in GB. Metaflumizone is a broad-spectrum insecticide used on various field crops to treat a wide range of pests such as Lepidoptera; Coleoptera; Hymenoptera. The approval of the active substance expired on 31 December 2024.
Extension of approval for glyphosate
The Glyphosate Renewal Group (GRG) has provided HSE with a dossier to support their application to renew the approval of glyphosate in GB. HSE has determined that the application is admissible and will now determine if glyphosate continues to meet the approval criteria for an active substance in GB.
To allow for a renewal assessment to be completed, HSE has extended the approval of glyphosate until 15 December 2026.
All PPP approval changes in GB since the beginning of 2025
Table 2 shows all changes to PPP approvals introduced in GB since the beginning of 2025.
Proposed future approval changes – WTO notifications
All draft regulations must be notified either to the WTO Sanitary and Phytosanitary Committee (SPS) or Technical Barriers Committee (TBT) for a commenting period of 60 days before they are adopted. This provides an important opportunity for countries to comment on the proposed changes, and to seek an amendment if a change is likely to have a significant impact on exports. To provide feedback stakeholders should contact their national WTO contact point .
Table 3 shows recent WTO notifications of draft regulations that propose further changes to EU active substance approvals.
References
Lewis, K.A., Tzilivakis, J., Warner, D. and Green, A. (2016). An international database for pesticide risk assessments and management. Human and Ecological Risk Assessment: An International Journal, 22(4): 1050-1064. DOI: 10.1080/10807039.2015.1133242
This publication has been developped by the Fit For Market Plus programme, implemented by COLEAD within the framework of Development cooperation between the Organisation of African, Caribbean and Pacific States (OACPS), and the European Union (EU).
This publication has been produced with the financial support of the EU and the OACPS. Its contents are the sole responsibility of COLEAD and can under no circumstances be regarded as reflecting the position of the EU or the OACPS.



