New procedure for notifying non-compliances to third countries in TRACES-NT
- 19/07/2022
- Posted by: Gaetan Dermien
- Category: ACP EN
Some weeks ago, the EU sent out a notice to let third countries know that the system for notifying plant health interceptions (via email) would be replaced with a new system that uses the TRACES-NT platform. It is important to disseminate this information to all the National Plant Protection Organisation (NPPO) officials that are responsible for the follow-up of shipments of plants and plant products to the EU (and any associated interceptions).
To access the new system, designated NPPO contact persons first need to be registered in TRACES-NT. Once registered, they are able to check whether there are any notifications relevant to their national exports. They can also monitor the import status of plant/plant product consignments entering the Union by accessing the entry documents (“CHED-PP”) that are issued by EU border control posts. These entry documents record the outcome of any official controls that have been carried out.
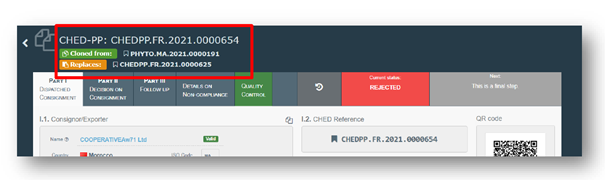
The latest changes were introduced in an amendment to the EU legislation[1], which was applied on 6 December 2021, accompanied by new developments in the use of TRACES-NT. EU border officials now record plant health interceptions in the entry documents using a new tab called “details on non-compliance”, alongside any decisions taken about the consignment.
Access to the CHED-PP module by NPPO contact points is in “read-only” mode (so no changes can be made), but it allows officials in exporting counties to monitor the status of their shipments in real time, and to take any necessary corrective action.
In the event of a rejection, the details of the non-compliance can be accessed via a separate tab in the CHED-PP module; this notes the reasons for the rejection and any actions taken regarding the consignment.
A User Manual is available to guide competent authority staff on the use of TRACES-NT; this explains how to access entry documents (CHED-PP) as well as the details of any non-compliances in the event of a rejected consignment.
The EC invites officials in non-EU countries to contact the TRACES support team at sante-traces@ec.europa.eu if they have any questions or concerns about using the new system.
Additional information and a video are also available on the EU website: TRACES (europa.eu)
[1] Regulation (EU) 2021/547 amending Implementing Regulation (EU) 2019/1715 as regards procedures for the establishment and use of ADIS and EUROPHYT, the issuance of electronic animal health certificates, official certificates, animal health/official certificates and commercial documents, the use of electronic signatures, and the functioning of TRACES, and repealing Decision 97/152/EC (https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32021R0547)


![EU and GB approval changes (January-May 2024) 9-FFM+-[ENG]](https://news.colead.link/wp-content/uploads/2024/06/9-FFM-ENG-150x150.jpg)

